 early Sleep Apnea Treatment in Stroke:
early Sleep Apnea Treatment in Stroke:
A Randomized, Rater-Blinded, Clinical Trial of Adaptive Servo-Ventilation
Supported by the Swiss National Science Foundation (grants 320030_149752 & 33IC30_166827)
Background and rational
The prevalence of Sleep Disordered Breathing (SDB) after acute stroke is high1). SDB after stroke has been found to be associated with a faster progression of stroke severity, higher blood pressure levels and longer hospitalization in the acute phase,2),3).
Chronically, stroke patients with SDB exhibit worse functional outcome4) and a higher mortality5). The mechanisms leading to the detrimental effects of SDB on stroke outcome are multiple and include changes of cerebral hemodynamics and brain oxygenation6) as well as a number of humoral and systemic changes7).
Due to the high prevalence of SDB following stroke and its detrimental effects on stroke outcome, it is crucial to investigate whether early treatment of central, obstructive and mixed forms of SDB with Adaptive Servo-Ventilation (ASV) has a beneficial effect on the evolution of the lesion volume and on stroke outcome.
Primary objective
The primary objective is to assess whether an immediate onset of ASV treatment in stroke patients with severe SDB (sSDB, Apnea-Hypopnea-Index (AHI) > 20) has a favorable effect on infarct growth assessed as the difference in lesion volume before and 90 days after stroke.
Eligibility criteria for stroke survivors
- Hospitalization on Stroke or Intermediate Care Unit
- Age 18-85
- Ischemic stroke detectible by neuroimaging, affecting the internal carotid artery or the anterior, middle or posterior cerebral artery (ACA, MCA or PCA) and/or branches thereof
- Symptom onset to admission < 24h
- AHI > 20 / h or < 5 / h
Exclusion criteria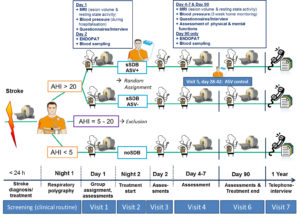
- Primary hemorrhagic stroke
- Secondary parenchymal haemorrhage (PH 1 and PH 2 according to ECASS)
- Small strokes (diameter <1.5 cm)
- Coma/Stupor
- Intubation
- Clinically unstable or life threatening condition
- Congestive heart failure (NYHA III-IV OR NYHA II and hospitalization due to CHF within the last 24 months OR LVEF ≤ 45%)
- Oxygen supply > 2 l/min during day and night
- Intermediate AHI value: ≥ 5/h and ≤ 20/h
- Known progressive neurological diseases
- Drug or alcohol abuse
- Inability to follow study procedure
- Pregnancy
- Any given contraindications to MRI or MRI-contrast agent
- Any given contraindications to ASV treatment
Study schedule and milestones
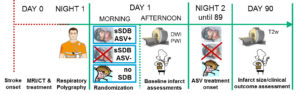
Stroke patients admitted to the hospital’s emergency unit must be continuously screened for study eligibility (criteria a.-e.). Acute evaluation of stroke etiology includes clinical assessment and performance of a standard stroke imaging protocol by MRI or CT (preferably MRI). Fulfillment of the AHI criterion f. must be assessed by a respiratory polygraphy performed during the first night after stroke. The next morning following stroke, patients with an AHI > 20 are randomized to ASV treatment or no treatment (sSDB ASV+ and sSDB ASV-). Patients without SDB (AHI < 5) are assigned to the comparison group. Patients with an AHI between 5 and 20 are not considered for study participation. This results in the following three study groups that are prospectively followed over 1 year:
- sSDB ASV+: stroke patients with an AHI > 20 randomized to ASV treatment
- sSDB ASV-: stroke patients with an AHI > 20 randomized to no treatment
- no SDB: stroke patients without SDB (AHI < 5)
ASV treatment starts the second night following stroke and ends 3 months later. Primary and secondary outcomes will be compared between these three patient groups.
MRI Assessments
Baseline assessments of the infarct volume and the volume of the infarct’s penumbra are performed in the afternoon at day 1 following stroke using Diffusion and Perfusion weighted imaging (DWI and PWI). These assessments are repeated during day 4-7 and 90 days following stroke. Final infarct volume at day 90 days is assessed on T2w MRI scans.
Volume assessments are performed by a treatment-blinded neuroradiologist. Contemporaneously, functional resting state magnetic resonance imaging (rsfMRI) is performed in all three groups of patients to assess the brain’s functional reorganization and recovery following stroke.
Stroke Severity, Clinical and Cognitive assessments
Stroke severity will be assessed by means of the NIHSS (stroke scale of the National Health Institute) on admission, day 1, days 4-7 and 3 months following stroke. Quality of life, clinical outcome (Barthel Index & modified Rankin Scale) and death from all causes and new cardio-cerebro-vascular events will be registered at 3 months and 1 year following stroke. At 3 months the sleep disordered breathing will be reassessed by a respiratory polygraphy.
To obtain a more precise assessment of cognitive and sensory-motor recovery, attention, memory, executive and sensorimotor functions will also be assessed with neuropsychological tests on days 4-7 and 3 months following stroke.
Physiological Assessments
The following physiological parameters will be assessed within the first weeks and three months after stroke:
- Blood pressure variability will be calculated based on 3 measurements a day over 3 weeks by an ambulatory automatic blood pressure device.
- Arterial stiffness and endothelial integrity will be assessed by recording arterial pulsatile volume changes in the fingertip with a non-invasive device named EndoPAT.
- Changes in standard and optionally also inflammatory blood parameters are monitored.
Sleep questionnaires & depression score
All participants will be interviewed regarding their pre- and post-stroke sleep history, and complete the Epworth sleepiness scale and the Berlin sleep apnea questionnaire on admission and at 3 months and 1 year following stroke. Moreover, depressive symptoms at 3 months and 1 year following stroke are assessed using Beck’s Depression Inventory II.
For collaborating hospitals and centers a more compact and less complex study schedule as illustrated in Figure 2 can be discussed.
Expected Outcome
- Beneficial effects of ASV-treatment 90 days following stroke are revealed by a smaller ischemic lesion extension and a higher resting state functional connectivity in large-scale cerebral networks measured by functional imaging and better stroke outcome.
- Beneficial effects of early onset ASV treatment in stroke survivors with SDB also manifest in improved clinical/cognitive outcome and hemodynamic parameters.
Relevance and Impact
The frequency of SBD in acute stroke is high (over 50% in more than 20 studies). Therefore, it is crucial to investigate whether treatment with ASV has a beneficial effect on the evolution of the ischemic lesion volume and thus neuronal plasticity processes, which are sensitive to hypoxia and hemodynamic instability. Evidence for a positive stroke outcome due to treatment with ASV and a better understanding of the detrimental effects of sSDB following acute stroke will change clinical practice and improve patients well-being.
References
1) Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke 2006;37:967-972.
2) Selic C, Siccoli M, Hermann DM, Bassetti CL. Blood pressure evolution after acute ischemic stroke in patients with and without sleep apnea. Stroke 2005;36:2614-2618.
3, 4) Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep 2003;26:293-297.
5) Bassetti C, Milanova M, Gugger M. Sleep disordered breathing and acute stroke: Diagnosis, risk factors, treatment, and longterm outcome. Stroke 2006;37:967-972.
6) Pizza F, Biallas M, Kallweit U, Wolf M, Bassetti CL. Cerebral hemodynamic changes in stroke during sleep-disordered breathing. Stroke 2012;43:1951-1953.
7) Cereda CW, Tamisier R, Manconi M, et al. Endothelial dysfunction and arterial stiffness in ischemic stroke: the role of sleep-disordered breathing. Stroke 2013;44:1175-1178.